Gemini:
The Power Grid of the Mind: Rethinking the Alzheimer’s Energy Crisis
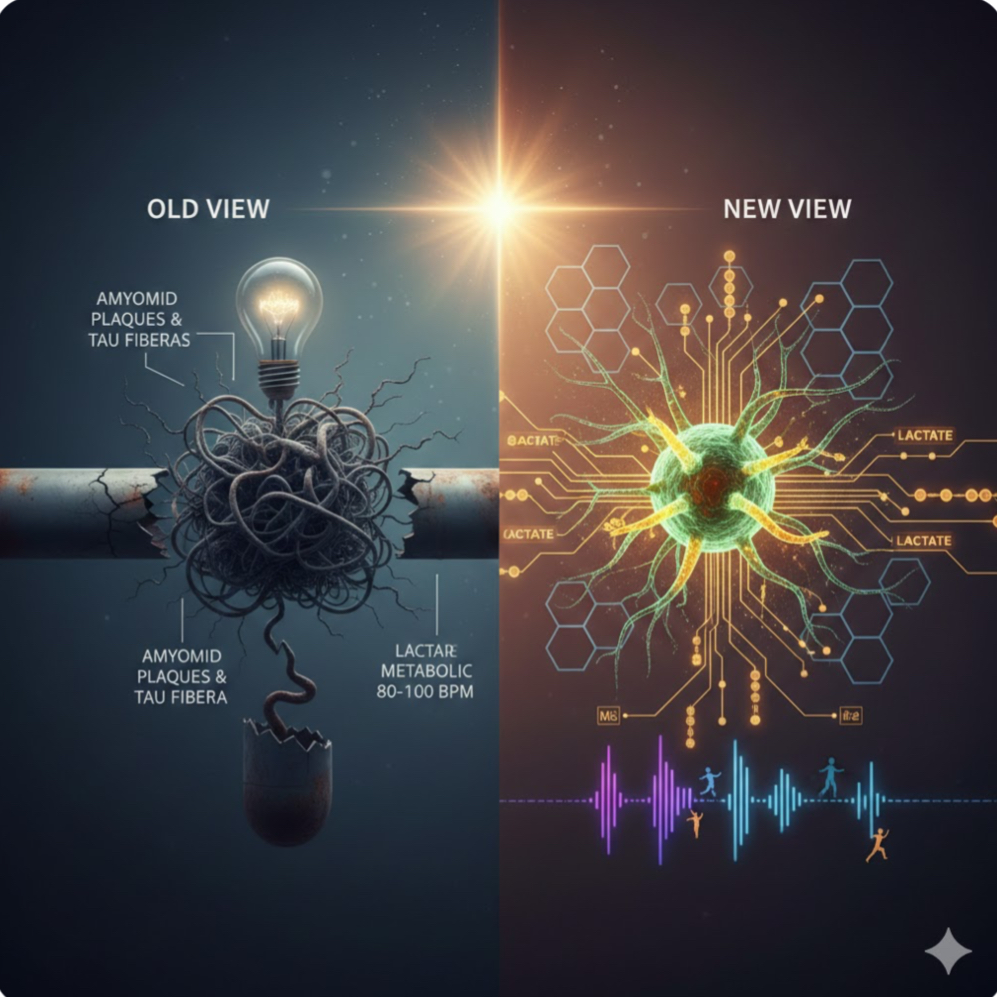
For over a century, the story of Alzheimer’s Disease (AD) was told through the lens of a “clogged pipe.” In 1906, Dr. Alois Alzheimer peered through his microscope and saw the wreckage of a mind: sticky clumps of protein (amyloid plaques) and tangled fibers (tau tangles). For decades, the prevailing logic was simple: clear the “trash,” and the brain will heal.
Yet, in 2025, we find ourselves at a crossroads. While modern medicine has finally succeeded in creating drugs that clear these plaques, the clinical results have been a sobering disappointment. Patients are losing their memories even when their brains appear “clean.” This mystery has fueled a revolutionary shift in neuroscience. We are moving away from the “Plumbing Hypothesis” and toward a far more dynamic understanding: The Energy Crisis Theory.
From Anatomy to Metabolism
The history of Alzheimer’s research has moved in waves. After the initial discovery of plaques, the 1970s brought the “Cholinergic Era,” which focused on a shortage of neurotransmitters. This led to the first generation of drugs, like Aricept, which managed symptoms but couldn’t stop the underlying decay. By the 1990s, the “Amyloid Cascade Hypothesis” dominated, fueled by genetic discoveries. Billions of dollars were poured into a single goal: stop the plaques.
However, as Kati Andreasson and other researchers at Stanford’s Wu Tsai Neurosciences Institute have recently highlighted, this focus may have been looking at the effect rather than the cause. We are now entering an era of “Systems Biology,” viewing Alzheimer’s not as a single protein failure, but as a multifactorial collapse of the brain’s metabolic infrastructure.
The Brain’s Power Grid: Astrocytes and Neurons
To understand this new perspective, we must look at how the brain feeds itself. Your brain is the most energy-demanding organ in your body. While neurons are the “stars” that send electrical signals, they are surprisingly bad at self-feeding. They rely on “helper cells” called astrocytes.
In a healthy brain, astrocytes act like a refinery: they take glucose from the blood, convert it into a high-octane fuel called lactate, and “hand it off” to the neurons. This “Lactate Shuttle” is essential for synaptic plasticity—the literal physical rewiring that occurs when we learn or remember.
Shutterstock
In Alzheimer’s, this power grid suffers a catastrophic failure. Research shows that chronic inflammation (often starting outside the brain) triggers an enzyme called IDO1. When IDO1 is overactive, it flips a metabolic switch inside the astrocytes, causing them to stop producing lactate. The result? The neurons don’t just “get sick”—they starve. This energy crisis explains why plaque-clearing drugs often fail: you can clean the trash off the streets, but if the power plant is dead, the city still won’t function.
The Parkinson’s Parallel
This “Energy Crisis” isn’t unique to Alzheimer’s. In Parkinson’s Disease (PD), a similar power failure occurs, but the location is different. While AD is a “fuel delivery” problem (the astrocyte fails), PD is often an “internal battery” problem. The mitochondria—the tiny engines inside the neurons themselves—break down.
Because the dopamine-producing neurons in Parkinson’s are some of the hardest-working cells in the human body, they are the first to die when the energy budget drops. Understanding both diseases as metabolic failures allows us to look for “universal” ways to rescue the brain’s power supply.
Re-Lighting the Brain: Exercise as Medicine
The most hopeful aspect of this metabolic shift is that it empowers us to take action through lifestyle. If the brain’s internal fueling system is broken, we can find “bypass” routes to deliver energy to starving neurons.
1. The High-Intensity “Bypass”: When you engage in high-intensity interval training (HIIT), your muscles produce lactate. This muscle-made lactate can cross the blood-brain barrier, acting as an “emergency fuel” that bypasses the broken astrocyte pathway. Just six minutes of vigorous activity has been shown to spike levels of brain-derived neurotrophic factor (BDNF), essentially “fertilizing” the brain’s connections.
2. Rhythmic Priming (80-100 BPM): Music is more than entertainment; it is a neurological tool. For those with motor or cognitive decline, music at a tempo of 80-100 beats per minute acts as an external “pacemaker.” This rhythm recruits healthy parts of the brain (like the cerebellum) to take over for damaged circuits, reducing the metabolic “cost” of movement and thought.
3. Nutritional Hybrid Fueling: Since an Alzheimer’s brain struggles to process glucose (leading some to call it “Type 3 Diabetes”), we can provide alternative fuels like ketones. Diets rich in healthy fats (like the MIND diet or MCT oil) provide a “backup generator” for neurons, allowing them to fire even when sugar metabolism is impaired.
A New Horizon
The shift from “plaques” to “power” is a watershed moment. We are no longer just looking for a “magic bullet” to kill a toxic protein; we are looking for ways to support the brain’s resilience. By combining cutting-edge pharmacology—such as repurposing cancer drugs (IDO1 inhibitors) to unlock astrocyte metabolism—with rhythmic music, specific exercise, and metabolic support, we are finally building a comprehensive strategy to fight neurodegeneration.
The goal is no longer just a “clean” brain, but a bright one—one where the lights stay on, the fuel keeps flowing, and the music never stops.
